Lung cancer vaccine trials begin across 7 countries including Türkiye
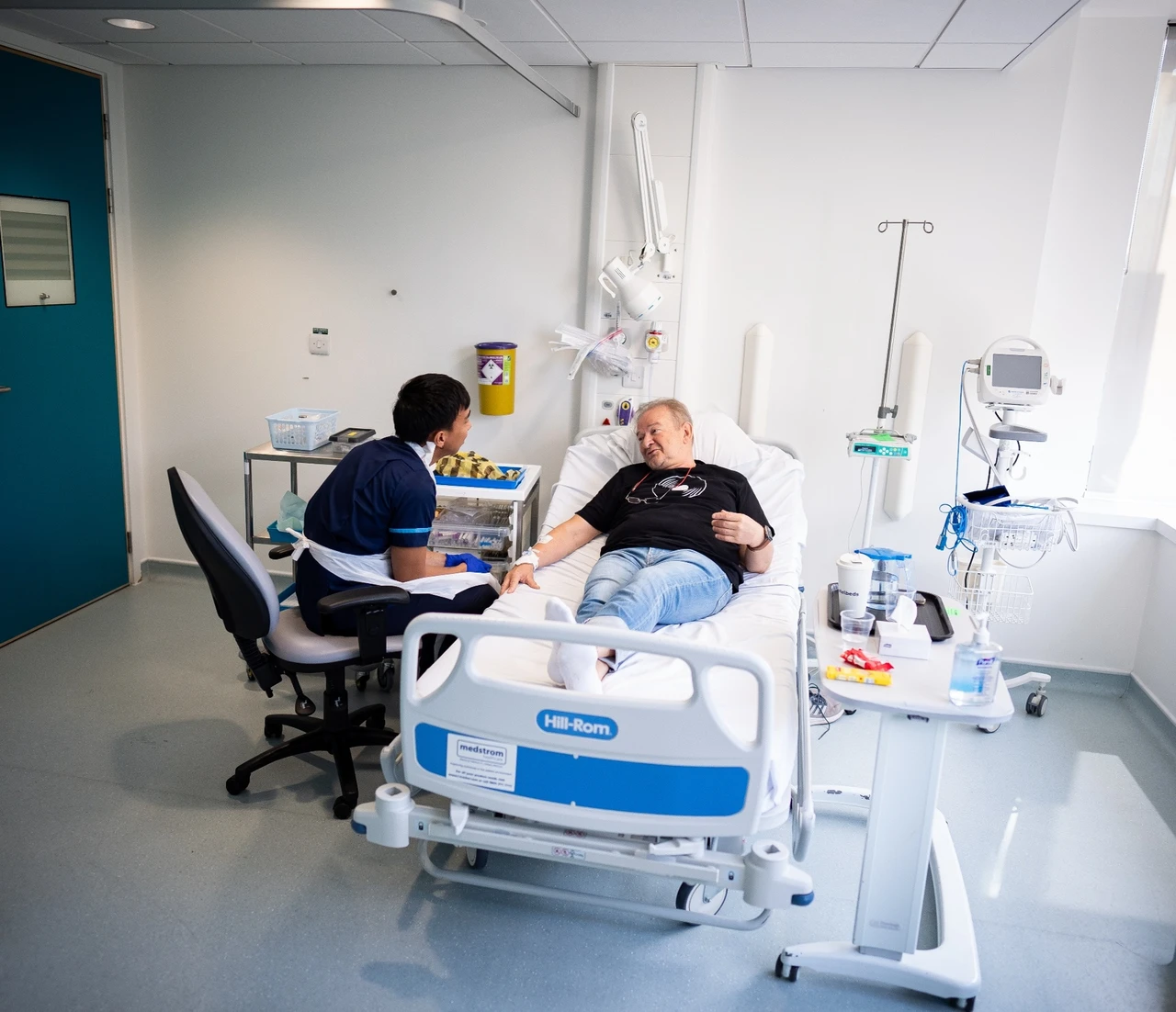 Senior research nurse Keenjee Nama with trial participant Janusz Racz. (Photo by Aaron Chown via PA)
Senior research nurse Keenjee Nama with trial participant Janusz Racz. (Photo by Aaron Chown via PA)
Doctors have commenced trials for the world’s first lung cancer vaccine using messenger RNA (mRNA), a treatment with the potential to revolutionize cancer care. The first patient in the U.K. received a dose of mRNA jab targeting non-small cell lung cancer.
The vaccine, BNT116, developed by BioNTech, is being tested in patients diagnosed with non-small cell lung cancer (NSCLC), the most prevalent form of the disease.
New era in cancer treatment
Lung cancer remains the leading cause of cancer deaths globally, accounting for approximately 1.8 million fatalities annually. The survival rates for advanced-stage lung cancer, where the cancer has spread, are particularly low.
This new vaccine aims to significantly improve these outcomes by instructing the body to hunt down and eliminate cancer cells while preventing their return.
The phase 1 clinical trial, marking the first human study of BNT116, is being conducted across 34 research sites in seven countries: the U.K., U.S., Germany, Hungary, Poland, Spain, and Türkiye. In the U.K., six sites are involved, with the first patient receiving the vaccine on Tuesday.
Targeting lung cancer with mRNA technology
BNT116 utilizes mRNA technology, similar to the COVID-19 vaccines, to present the immune system with tumor markers specific to NSCLC. This primes the body to attack cancer cells expressing these markers, aiming to bolster the immune response against cancer while leaving healthy cells unharmed, unlike traditional chemotherapy.
Professor Siow Ming Lee, a consultant medical oncologist at University College London Hospitals NHS Foundation Trust (UCLH), which is leading the trial in the U.K., stated, “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer. This technology is the next big phase of cancer treatment.”
First UK patient: Scientist’s journey
Janusz Racz, a 67-year-old scientist specializing in AI from London, became the first person in the U.K. to receive the vaccine. Diagnosed in May, Racz began chemotherapy and radiotherapy soon after. His scientific background inspired him to participate in the trial.
I am a scientist too, and I understand that the progress of science – especially in medicine – lies in people agreeing to be involved in such investigations
Janusz Racz
Racz received six consecutive injections, each five minutes apart, over 30 minutes at the National Institute for Health Research UCLH Clinical Research Facility. The vaccine regimen includes weekly doses for six weeks, followed by a dose every three weeks for 54 weeks.

Potential for lifesaving impact
Professor Lee emphasized the trial’s importance, stating, “We hope adding this additional treatment will stop the cancer from coming back because, a lot of the time for lung cancer patients, even after surgery and radiation, it does come back.”
As the trial progresses, experts are hopeful that the mRNA vaccine, in combination with immunotherapy, could significantly improve survival rates. If successful, BNT116 may proceed to phase 2 and 3 trials, potentially becoming a standard of care for lung cancer patients worldwide.



