WHO approves first Mpox vaccine, urges immediate action in Africa
 A test tube labelled "mpox virus positive" is held in this illustration taken Aug. 20, 2024. (Reuters Photo)
A test tube labelled "mpox virus positive" is held in this illustration taken Aug. 20, 2024. (Reuters Photo)
The World Health Organization (WHO) announced that “MVA-BN,” produced by Bavarian Nordic, is the first vaccine to receive prequalification against the Mpox virus.
WHO‘s statement noted that the vaccine‘s approval will help provide timely access to communities in urgent need, reduce transmission, and assist in controlling outbreaks.
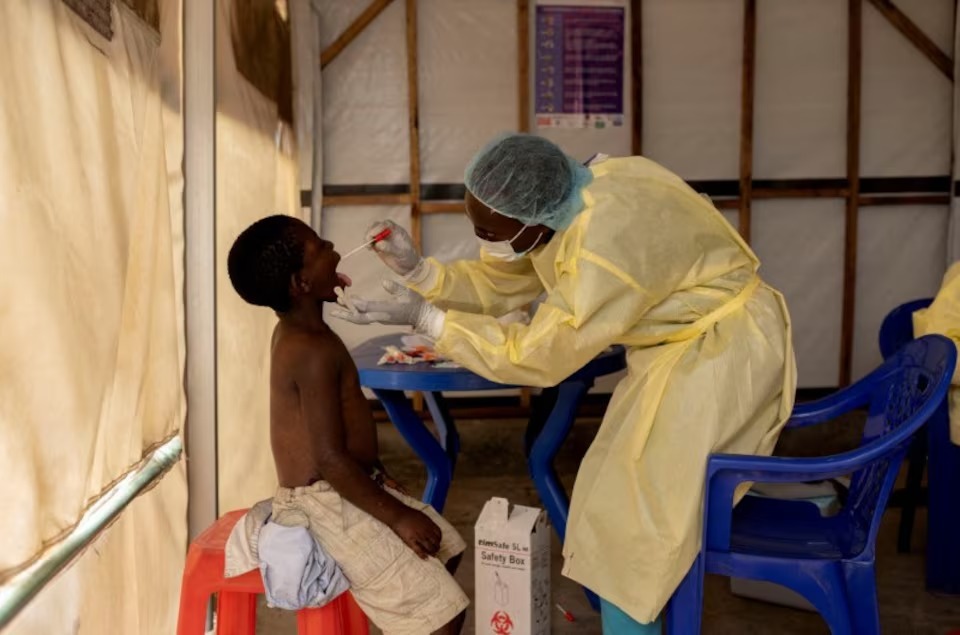
2 dozes, four weeks apart
The Strategic Advisory Group of Experts (SAGE) reviewed the evidence and recommended MVA-BN for high-risk individuals. It can be administered in two doses, four weeks apart, to those over 18.
While not licensed for individuals under 18, the vaccine can be used off-label for infants, children, adolescents, pregnant women, and the immunocompromised.
WHO Director-General Tedros Adhanom Ghebreyesus emphasized the need for urgent production, supply, donation, and distribution to ensure equitable access, especially in regions like Africa.
The Mpox virus is transmitted through contact with infected animals or individuals. Transmission occurs by touching rashes, contaminated clothing, or bodily fluids. Symptoms appear 5 to 21 days after exposure and include fever, headaches, muscle pain, swollen lymph nodes, and chickenpox-like blisters. The disease is treated with antiviral drugs, and most cases recover within weeks.
In 2022, WHO renamed “monkeypox” to “mpox” due to concerns over racism and discrimination. The virus was declared a “public health emergency of international concern” on August 14.



