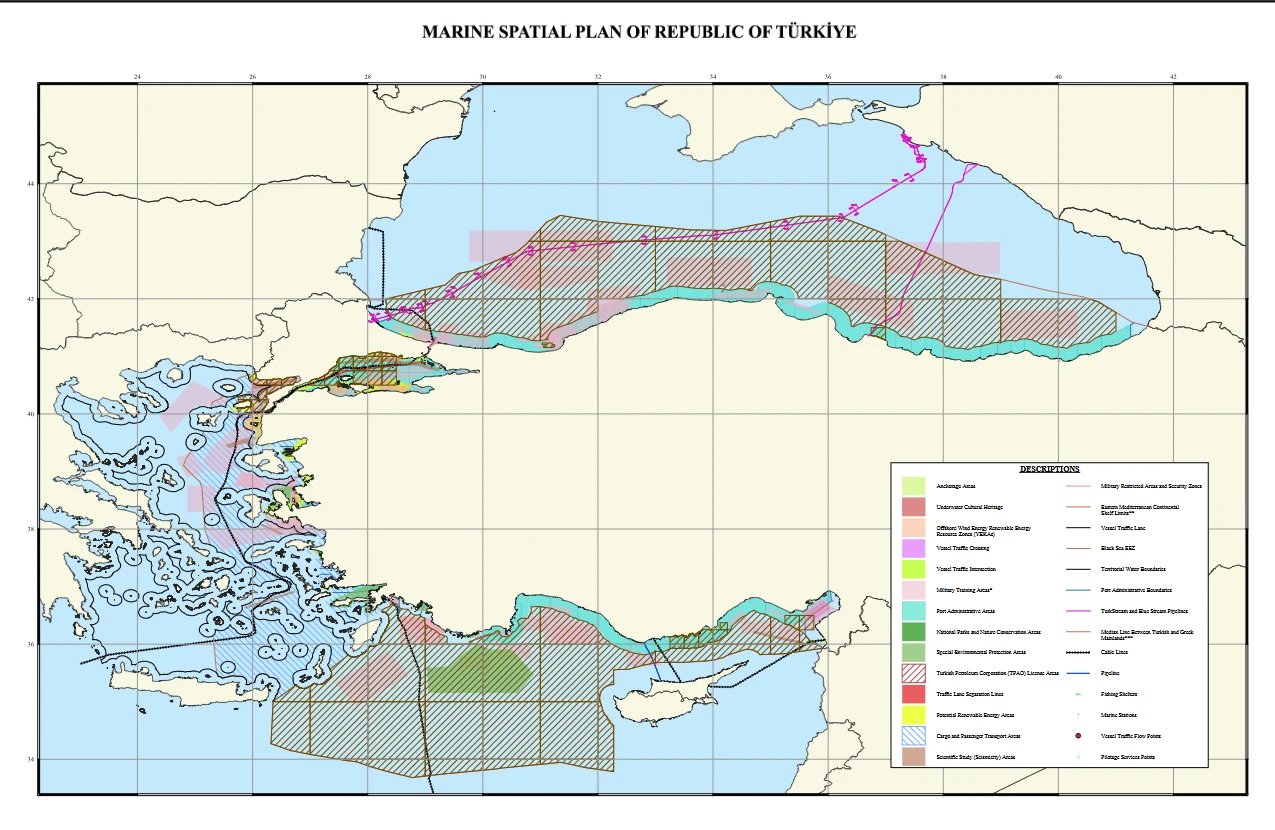
China launches clinical trials for its first mpox vaccine
 A monkeypox vaccine shown in August 2022. (Photo via Media News Group via Getty Images)
A monkeypox vaccine shown in August 2022. (Photo via Media News Group via Getty Images)
China has begun clinical trials for its first domestically developed mpox vaccine, with state-run media reporting on Wednesday amid rising global concerns over the virus’ spread.
The vaccine, created by the Shanghai Institute of Biological Products Co. (SIBP), a branch of China National Pharmaceutical Group Corporation (Sinopharm), will undergo phase 1 trials at the Henan Infection Diseases Hospital in Zhengzhou Henan province, according to Global Times.
The first phase of the trial plans to enroll volunteers aged 18 and older from the area. The vaccine received approval for clinical trials from China’s National Medical Products Administration in September 2024.
The World Health Organization (WHO) declared the outbreak of a new clade 1 variant of mpox a public health emergency of international concern in August 2024, following its rapid spread in several African countries.
According to the WHO, as of March 30, 23 countries have reported 24,177 confirmed mpox cases and 98 deaths over the past 12 months. The Democratic Republic of the Congo has reported the most cases (14,566), followed by Uganda (4,881) and Burundi (3,725).